Difference between Osmosis and Diffusion?
Osmosis-
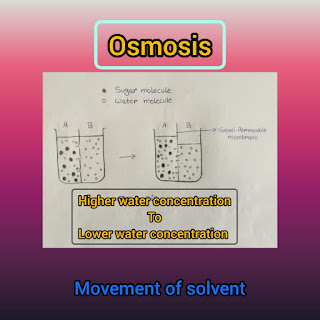
1) It is the movement of solvent from higher water concentration to lower water concentration .
2) It take place through semi permeable membrane.
3) Osmosis occurs in liquid medium only.
4) Only the solvent molecules move from one place to another.
5) It does not equalize the concentration of solvent molecules.
6) Osmosis can be stopped and reversed by applying external pressure.
7) Example- Sugar molecules are solute and the water molecules are solvent. This example shows the movement of water molecules (solvent) from higher concentration to lower concentration.
Diffusion-
1) It is the movement of solute from higher concentration to the lower concentration.
2) This process does not require semi-permeable membrane.
3) Diffusion occur in any medium solid, liquid or gas.
4) Solute and solvent molecules both can move from one place to another.
5) It equalizes the concentration of solute molecules.
6) Diffusion process cannot be stopped or reversed.
7) Example- The sugar molecules are solute and the water molecules are solvent. This example shows the movement of solute (sugar molecules) from higher concentration to lower concentration.




.jpeg)
Comments
Post a Comment